
On this World Blood Donor Day, let’s explore some Israeli tech that is making a difference to one of our most vital components: our blood
Hematological awareness is important as body vitality is exclusively dependent on blood flow. Blood is our natural producer of oxygen and carbon dioxide, and it is also a key mechanism for medical identification. For decades, doctors have prioritized blood analytics and testing as these results present in-depth information regarding diagnostics, treatment methods, and one’s overall physical state. Blood is as much the cure as it is the cause– blood donations are organized worldwide to act as a remedy for the shortage of blood and blood products that can single-handedly save hundreds of lives. Here in Israel, MedTech’s innovative efforts have always been advanced, yet amidst the effects of COVID-19 and how it continues to modify all spheres of society, Israeli MedTech innovations are at an all-time high. With the focus strictly on blood analytics, Israel’s start-up environment has pushed forth incentives and actions that have seemingly revolutionized how blood can be analyzed, detected, and measured. Here are some Israeli startups that play a role in hematology solutions.
PixCell
Blood diagnostic information, in most cases, does not do enough to create an effective impact on immediate health solutions and products. The devices and technologies that have been used thus far do not provide doctors and patients with substantial information when it matters most. Moreover, real-time blood testing is not accessible to everyone which presents numerous implications when patients are in emergencies. These obstacles have disrupted medical efforts for decades as there is a pressing need for in-depth, easily accessible blood diagnostic tools. In an accidental discovery made by a team of researchers at the Technion (Israel Institute of Technology), Viscoelastic Focusing (VEF) has been recognized as the saving grace toward efficient cell analytics. The Israeli startup PixCell has created the HemoScreen that uses advanced technology making use of VEF potential. This product uses a disposable cartridge with specific reagents to elicit a Complete Blood Count test. HemoScreen does not require maintenance or calibration and is versatile to fit the precise needs of its user.

PixCell was founded in 2008 with a keen focus on simplifying and regularizing real-time blood testing worldwide. Their research and entrepreneurial staff have surpassed company expectations with the discovery of Viscoelastic Focusing (VEF), paving a way for pivotal advancements in hematological efforts. Their team consists of Avishay Bransky (CEO, Co-Founder), Prof. Max Herzberg (Co-Founder), Armin Schon (CCO), Hanan Ben-Asher (COO, BD Manager), Mark Erez (CFO), Yaara Ben-Yosef (Director of RA and Clinical Affairs), and Eitan Hod (Director of QA and RA). PixCell has raised over $7 million in grant funding from the European Commission, the International Health-Tech Pilot Program, and the Israel Innovation Authority. In 2021 PixCell was acquired by Soulbrain Holdings.
RedC Biotech
The global supply of red blood cells has seeped far under worldwide medical demands. This shortage has served as a detriment to medical interventions such as trauma, childbirth, operations, chronic illness, cancer, and the list goes on. Red blood cells are the oxygen-carrying capacity of the body which is why 120 million blood units are donated each year, yet there is still a concerning decrease in these donations. Blood drives and donation sites, although they function as a temporary solution, are still not enough to compensate for the urgency in transfusion methods, productions, and resources. The Israeli startup RedC Biotech has initiated a “one blood type fits all” method with RedC Universal Red Blood Cell Transfusions. This product is suitable for almost every patient and provides a uniform potency. It is pathogen-free and donor free and also eliminates undesirable blood components as well as additional hospital testing.

RedC was founded in Haifa in 2015 by Dr. Ari Gargir. Over the past couple of years, the company has received $1.4 million in pre-seed and seed funding from PipelBiz. Their revolutionary technology addresses issues associated with industrial red blood cell production, and cost reductions. From the lab to global production, Red C Biotech is eager to scale the count of red blood cells to save lives worldwide. RedC has a small employee base but is growing in accordance with its production timeline.
Improdia
Current diagnostic tests cannot distinguish between acute and chronic inflammation, as well as monitoring immune functions that are pivotal for personalized treatment methods. With Improdia’s array of Unique BioMarkers, the innovative diagnostic company has developed a simple way to supervise inflammation levels for patients suffering from autoimmune and cancer diseases. ImproDdia’s technology department has made substantial discoveries enabling pharma companies a kickstart in their drug development processes as well as suggesting personalized treatment methods. Improdia’s Diagnostic Kit includes three easy-to-use, unique biomarkers with three distinct functions: the IMPC (for chronic inflammation), IMPI (for immunotherapy), and IMPD (for diabetes complications. IMPC evaluates the immune status and inflammatory pain levels of those with niche medical implications. It aids physicians in selecting the type and timing of specific treatments as well as monitoring the efficacy of such treatments. IMPI is a prognostic test for the efficiency of immunotherapy. It monitors the immune status of cancer patients before immunotherapy or chemotherapy. IMPD is a prognostic test for diabetes complications as it predicts the occurrence of these complications before they are medically obvious.

Improdia’s team consists of Miriam Lerner (Founder, Co-CEO, CTO), Gil Pogozelich (Chairman), Dr. Roy Eldor (Medical Director), Prof. Michal Baniyash (Inventor), and Prof. Ido Wolf (Member of the Scientific Advisory Board). The company has been based in Herzliya since 2012 and continues to aid doctors and patients in the diagnostic and management endeavours of those with chronic immune-mediated diseases.
Sight Diagnostics
Sight’s technology represents breakthrough innovations in diagnostic methodology. Their latest blood analyzer, Sight OLO, performs a Complete Blood Count, the most ordered blood test, in minutes. It’s compact and designed to be used in a variety of settings. OLO creates a digital version of a blood sample by capturing more than 1,000 highly detailed images from just two drops of blood obtained from a finger prick or venous sample. These images are then interpreted by proprietary and fully automated AI algorithms, and the results are available within minutes.
Sight OLO provides 5-part differential complete blood count (CBC) results with 19 parameters and sophisticated flagging capabilities, for on-site testing. It is the first CBC analyzer that is FDA 510(k) cleared for blood taken directly from either a finger prick or a venous sample. Sight OLO has been validated for use in patients 3 months and above, in a variety of CLIA-certified (Clinical Laboratory Improvement Amendments) moderately complex clinical settings such as hospitals, emergency departments, oncology clinics, pediatric practices, and urgent care locations. The sample preparation process can be completed in under one minute, with the full results ready in minutes on a touchscreen interface, printout, email, or via LIS (Latent Semantic Indexing)/middleware. Sight OLO comes factory calibrated for a quick setup with internal Failsafe systems and requires no maintenance. Its minimal training and step-by-step on-screen guidance are designed to be used by operators with any level of experience. OLO also has an Operator Management feature that allows for complete control over who can access the device, including traceability of operator activities.

Sight OLO was founded in 2011 by Yossi Pollack (CEO) and Daniel Levner (Chairman of the Scientific Advisory Board). The company is based in Tel Aviv, London, and Brooklyn with 300 employees. With investments from Koch Disruptive Technologies, OurCrowd, and Longliv, Sight Diagnostics has raised over $120 million to date. Sight has gained worldwide traction with partners eager to use and expand the company’s advanced technology. Among them are Boston Children’s Hospital, Oxford University Hospitals NHS Foundation Trust, and Nicklaus Children’s Hospital in Miami.
EFA
Handheld blood test diagnostic tools are not affordable for the average patient and consumer. Most of these tools require laboratory conditions and specific preparations which fail to account for real-time informed decisions an individual may have to make. Israeli startup EFA developed RevDx™, a revolutionary, European Commission-approved, mobile, and fully automated diagnostic system to be used for different care, including primary care physicians, home care, emergency care, and remote care. RevDx™ is easy-to-use; from a finger prick, you get results within minutes. The application provides a Complete Blood Count test, the most requested hematology test worldwide which provides indications for broad clinical conditions such as infections, disease, fever, immune system, and anemia.

Founded in 2016 by Yoel Ezra (CEO), an engineer and a physicist who served in a technological-operation unit in the IDF for over 23 years, EFA is acting to develop the RevDx solution as a platform that will enable the creation of more diagnostic applications over time. Among the company’s investors are Maccabi Healthcare Services, eHealth Ventures, Merchavia, and Medison. The company currently employs 12 people.
OrSense
Diverse clinical settings have not found easy nor accurate methods to measure blood parameters as current testing devices and tools are not appropriately equipped for such conditions. The key to medical testing is to establish a comfortable environment for any and all patients; with invasive monitoring, though, this element is rather ignored. Israeli startup OrSense works to transform patient health through non-invasive monitoring as they develop and commercialize innovative monitoring technologies focused primarily on donor and patient comfort. Using their revolutionary SpectOLight ™ Occlusion Spectroscopy Technology, OrSense has developed the NBM 200 Device that detects blood hemoglobin (Hb), oxygen saturation levels and pulse rate (PR) values. Patients place their fingers on a ring-sensor probe where the portable desktop attached to the monitor device calculates and displays the figured measurements. To make matters even easier and more efficient, OrSense has developed a software application attached to the device that allows for Tablet or Smartphone use.
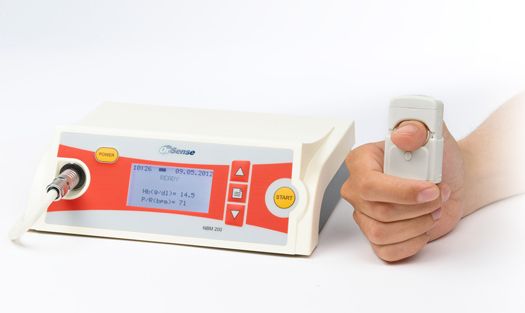
Since 1996 OrSense has been working worldwide to overcome key obstacles that do not account for comfortable, accessible, and efficient blood parameter testing. For over 20 years, OrSense has made substantial adjustments toward non-invasive monitoring technologies: the intellectual property portfolio at OrSense consists of 51 granted patents and over 20 additional applications. So far, OrSense has raised over $44.4 million in seed and grant funding from Israel HealthCare Ventures, Star Ventures, Saints Capital, The Lewis Trust Group, and Shimon Eckhouse. Their team consists of Yoav Resiman (Founder and CEO), Aharon Weinstein (VP of Research). Asher Zysman (CFO), and Chip Neff (President). OrSense is located both in Tel Aviv, and Raleigh, North Carolina, USA.
PatenSee
Patients who suffer from renal failure are required to be connected to a hemodialysis machine for a few hours, 2-3 times a week, via a fistula on the patient’s arm. The fistula is the point of connection between the hemodialysis machine and the patient for the treatment. One of the most dreaded complications for hemodialysis patients is fistula loss due to stenosis (blood clogging of the fistula) resulting in an inability to perform routine dialysis – a major risk to patient life. PatenSee is a medical device company (currently in the clinical stage) that has developed a contactless, machine vision-based, surveillance system for the early detection of vascular stenosis and/or a clogged fistula. The technology is designed to provide a very simple way to improve the quality of patient care and to support the nursing staff in the hemodialysis center without adding any burden on the patient or the clinic. The system can also be adapted to home use adding a critical diagnostic feature to home dialysis.
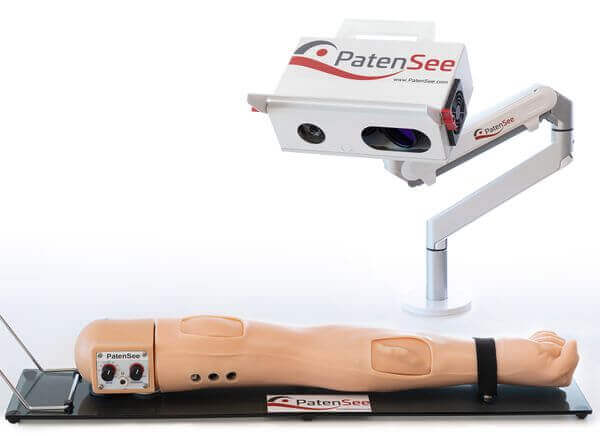
PatenSee was founded in 2019 as a portfolio company of MEDX Xelerator, a MedTech incubator working under a license from the Israel Innovation Authority. Hagay Drori and Oz Seadia founded PatenSee based on an unmet need presented to the MEDX Xelerator by a major strategic player in the dialysis field. Dr. Gal Goshen, PatenSee’s CEO, led it from its early concept through its first-in-human 60-patient clinical study, conducted less than two years after the company’s founding. PatenSee is currently raising a Series A round and is preparing for a multi-center, international, clinical trial of its second-generation imaging system.
